Modules
Stage One
| Code | Title | Status | Action |
|---|---|---|---|
| Code HLS020 | Title Introduction to Haematology | Status Compulsory | Action View |
| Code HLS021 | Title Laboratory Haematology | Status Compulsory | Action View |
| Code HLS022 | Title Health and safety in haematology practice | Status Compulsory | Action View |
| Code HLS023 | Title Anaemia | Status Compulsory | Action View |
| Code HLS024 | Title Acute leukaemia | Status Compulsory | Action View |
| Code HLS025 | Title Chronic leukaemia | Status Compulsory | Action View |
| Code HLS026 | Title Myeloma | Status Compulsory | Action View |
| Code HLS027 | Title Congenital coagulation disorders | Status Compulsory | Action View |
| Code HLS028 | Title Thrombophilia | Status Compulsory | Action View |
| Code HLS029 | Title Anticoagulation | Status Compulsory | Action View |
| Code HLS030 | Title Acquired bleeding disorders | Status Compulsory | Action View |
| Code HLS031 | Title Bone marrow failure syndromes 1 | Status Compulsory | Action View |
| Code HLS032 | Title Haemoglobinopathy | Status Compulsory | Action View |
| Code HLS033 | Title Myeloproliferative disorders | Status Compulsory | Action View |
| Code HLS034 | Title Transfusion medicine | Status Compulsory | Action View |
| Code HLS035 | Title Haematology relating to other medical specialities and communication skills | Status Compulsory | Action View |
| Code HLS036 | Title Lymphoma 1 | Status Compulsory | Action View |
| Code HLS037 | Title Platelet disorders 1 | Status Compulsory | Action View |
Stage Two
| Code | Title | Status | Action |
|---|---|---|---|
| Code HCC320 | Title Developing independent professional practice | Status Compulsory | Action View |
| Code HCC321 | Title Research, development and innovation | Status Compulsory | Action View |
| Code HCC322 | Title Training and education | Status Compulsory | Action View |
| Code HCC323 | Title Leadership and management | Status Compulsory | Action View |
| Code HLS150 | Title Haematology Blood transfusion | Status Compulsory | Action View |
| Code HLS153 | Title Haemostatic pathways and principles of coagulation | Status Compulsory | Action View |
| Code HLS154 | Title General laboratory investigation | Status Compulsory | Action View |
| Code HLS155 | Title Bleeding disorders haemophilia A and haemophilia B | Status Compulsory | Action View |
| Code HLS156 | Title Bleeding disorders Von Willebrand disease | Status Compulsory | Action View |
| Code HLS157 | Title Other bleeding disorders | Status Compulsory | Action View |
| Code HLS158 | Title Thrombotic disorders | Status Compulsory | Action View |
| Code HLS159 | Title Anticoagulant therapy and prophylaxis | Status Compulsory | Action View |
| Code HLS160 | Title Haematological malignancy | Status Compulsory | Action View |
| Code HLS175 | Title Molecular diagnostics -Haemostasis and thrombosis | Status Compulsory | Action View |
| Code HLS177 | Title Platelet disorders 2 | Status Compulsory | Action View |
Programme specification
Details
- Programme title
- Higher Specialist Scientist Training Programme (HSST) — Haematology (Haemostasis and Thrombosis)
- Division
- Life Sciences
- Normal length of programme
- 5 years
- Outcome awards and professional eligibility
-
Successful candidates will be awarded:
- Certificate of Completion of the Higher Specialist Scientist Training Programme. Awarded by the National School of Healthcare Science.
- Total credits
- 540
- Mode of study
- Work-based with integrated part time DClinSci or FRCPath
- Programme accredited by
- The Academy for Healthcare Science
Programme aim
The programme has been developed to enable a selected cohort of clinical scientists to be trained to take on the role of a consultant clinical scientist. It is a programme that is both flexible and bespoke to the individual.
It includes the incorporation into the training programme of all or part of a professional doctorate award according to trainee previous experience and qualifications and comprising modules in:
- High level scientific and clinical knowledge
- Leadership and professionalism
- Research and innovation
Expected programme outcomes
On successful completion of the HSST and its underpinning doctoral level academic programme graduates will possess the essential knowledge, skills, experience and attributes required of a Consultant Clinical Scientist in the NHS and will:
- demonstrate practice that places the patient and the public at the centre of care, prioritising patient safety and dignity and reflecting NHS/health service values, the NHS Constitution and meets the professional standards defined by Good Scientific Practice;
- demonstrate systematic acquisition and understanding of a substantial body of scientific and clinical knowledge which is at the forefront of healthcare science;
- possess a breadth of clinical and scientific knowledge across a range of related and relevant science specialties;
- demonstrate the general ability to conceptualise, design, lead and implement a project for the generation of new knowledge, applications or understanding at the forefront of healthcare science;
- demonstrate an advanced and detailed understanding of applicable methods for research, innovation and advanced academic enquiry;
- demonstrate the ability to create and interpret new knowledge, through original research or other advanced scholarship, of a quality to satisfy peer review, extend the forefront of the healthcare science/specialism, and merit publication;
- demonstrate advanced critical thinking with a sound grasp and application of research methodology which supports vision and innovation in the application of basic science to health and an effective understanding of leadership, NHS current policy, influencing and advanced communication skills;
- communicate clearly to specialist and non-specialist audiences including patients and the public;
- demonstrate scientific and clinical leadership based on the continual advancement of their knowledge, skills and understanding through the independent learning required for continuing professional development.
Will be able to:
- make informed judgements on complex issues in specialist fields, often in the absence of complete data, and be able to communicate their ideas and conclusions clearly and effectively to specialist and non-specialist audiences;
- deal with uncertainty;
- continue to engage in continuous personal and professional development, resulting in leading the development of new techniques, ensuring innovation and facilitation of transformational change in science and in the service through ideas or approaches that benefit the patient and NHS;
- engage with contemporary research and analysis as a basis for critical reflection on their own and others’ professional experience and work based practice.
Will have:
- the qualities and transferable skills necessary for employment as a Consultant Clinical Scientist, which requires the exercise of personal responsibility and largely autonomous initiative in complex and unpredictable situations, in professional or equivalent environments leading and working within teams
Learning and teaching methods
A blend of methods is used to ensure effective capture of robust evidence relating to the three components of the HSST programme.
The components of the programme are:
- Acquisition and application of specialist scientific knowledge up to Consultant Clinical Scientist level.
- Mastery and practice of higher clinical and scientific skills, including professionalism, and the values, behaviours and attitudes expected of leaders in healthcare science.
- Ability to make a significant contribution to knowledge generation and innovation in healthcare science service and practice.
Assessment methods
- Portfolio of workplace-based evidence
- Annual Review of Progression
- Multi Source Feedback
- Independent Assessment of Professional Skills (IAPS) – HSST Final Assessment
Standards of proficiency
To gain entry on to the Higher Specialist Scientist Register you must demonstrate that you are able to practice within the 5 Domains:
- Domain One: Professional Practice
- Domain Two: Scientific Practice
- Domain Three: Clinical Practice
- Domain Four: Research, Development and Innovation
- Domain Five: Clinical Leadership
Programme structure
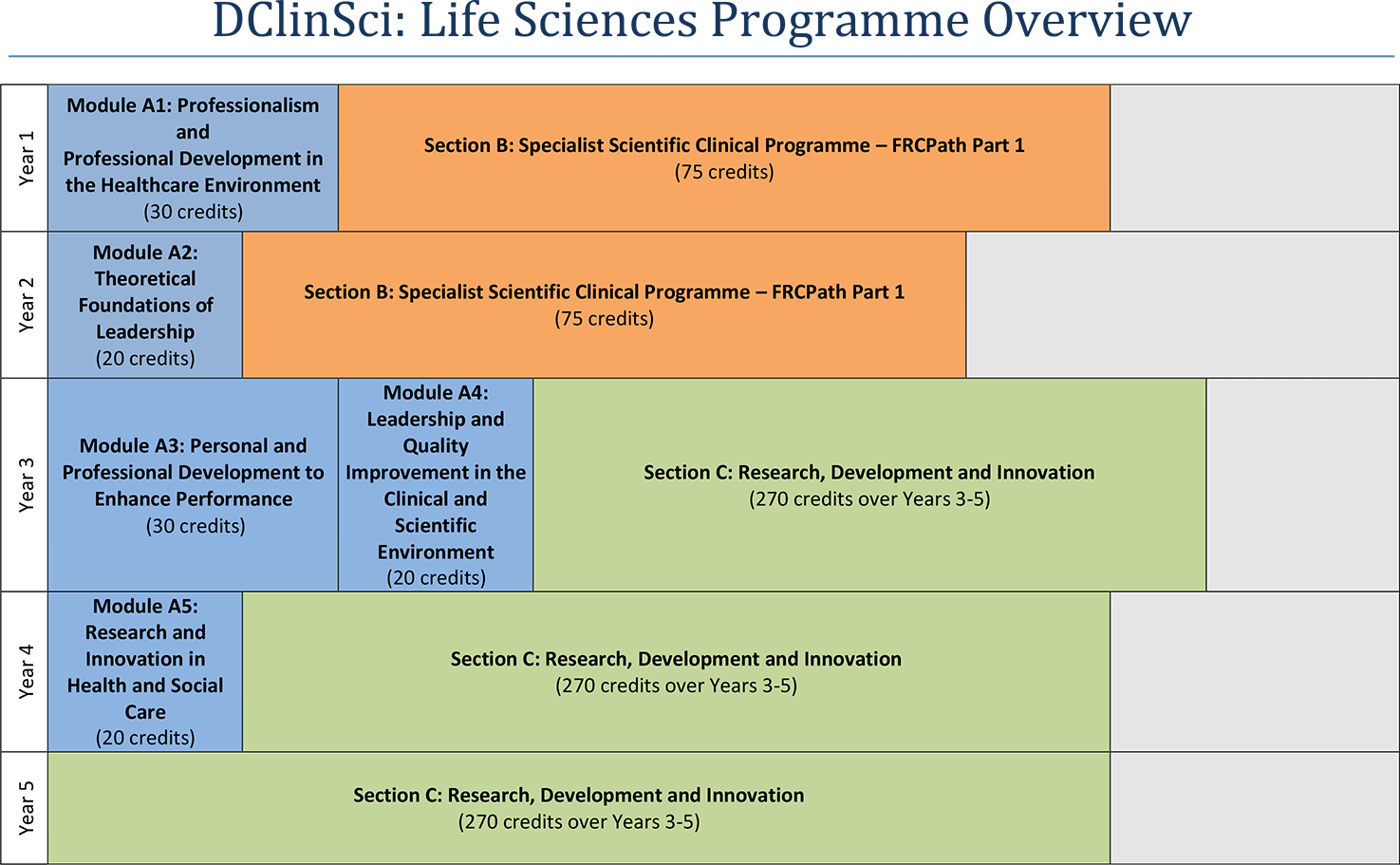
Entry routes and requirements
| Entry routes | In England there are two routes into the programme
|
| Entry requirements | For both entry routes, HSST applicants must participate in the national recruitment/assessment process and meet the minimum entry requirements for the academic and work-based programme.
More detailed information on entry routes and requirements can be found on the NSHCS website. |